
The Role of Dendritic Cells in the Maturation of T Cells
DANIEL ALLENDORF
IMMUNOLOGY 413
SEPTMBER 23, 1999
Dendritic cells (DC) are an important component of the humoral immune response.1 Dendritic cells are the most prevalent and most efficient of a class of cells called antigen presenting cells (APC).1,2,3 As an APC, it is the responsibility of DC to interact with T cells in lymphoidal tissues and activate them from a naive state to an effector state.1,2,3,4,5,6,7,8,9
Dendritic Cell Origins
DC originate from the common myeloid progenitor of white blood cells. Myeloid cells are created in the bone marrow and then travel to the periphery as specialized cells. More specifically, DC come from monocytes.8 It is currently suggested that when a monocyte leaves the bloodstream, engulfs an antigenic particle in peripheral tissues, and migrates toward the lymphatic system it differentiates into a DC.8 This voyage through the periphery must occur from a deep to superficial direction called reverse transmigration.8 Those monocytes that do not participate in reverse transmigration usually become macrophages.8
T Cell Origins
T cells derive from a lymphoid progenitor also common to B cells and natural killer (NK) cells in the bone marrow.1 T cells eventually migrate to the thymus gland, anterior and slightly superior to the heart, and undergo differentiation. Here, T cells process those cells that will recognize either self or foreign cells. Those cells that are likely to recognize self cells and elicit an autoimmune response are destroyed by apoptosis through a process called negative selection, in which the DC play a pivotal role.1 T cells then go on to participate in cell-mediated immunity in which they are activated by DC and differentiate into cytotoxic T cells (Tc) or T helper cells (TH).1,2,3,4,5,6,7,8
Dendritic Cells as Antigen Presenting Cells (APC)
DC can exist in either of two forms: immature or mature. Immature DC reside outside the lymphoidal tissues in peripheral areas, especially on the epithelial surfaces of hard organs or in the skin. DC are active in phago-/pinocytes and upon ingesting a pathogen, or any antigen (Ag) containing substance, will process and degrade the pathogen in such a way that peptide fragments of Ag will join with the major histocompatability complex type I or II (MHC 1 & II), from the trans Golgi network, and migrate to the cell surface for presentation.1,2,4,5,7,8,9 Antigens bind to MHC I if they are derived from the cytoplasm of the infected cell. The MHC I is presented to those T cells displaying the CD8 antigen binding site. These cells are then activated and will become Tc cells.
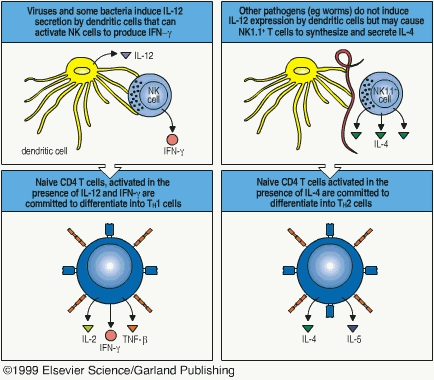
Antigen binds to MHC II if it is derived from an intracellular vesicle, toxin, or extracellular microorganism. MHC II is presented to T cells displaying the CD4 antigen binding site. These cells then become TH cells either type 1 or 2.1 TH1 are formed from CD4-MHC II interactions in the presence of IL-12 or IFN-g.1 TH2 cells are formed from the CD4-MHC II interaction in the presence of IL-4. The "decision" to secrete these cytokines is chemical in nature and is dependent on the strength of the bond between CD4-MHC II.1


Fig. 8.26 & 8.30 also illustrate this very well…
Upon presenting Ag, a DC is mature. Mature DC then migrate to lymphoidal tissues. Ag is presented by DC to naive T cells within lymphoidal tissue as described.1 Then, mature DC most likely die within the lymphatic tissue as there is no vestige of them in efferent lymphatic ducts.8
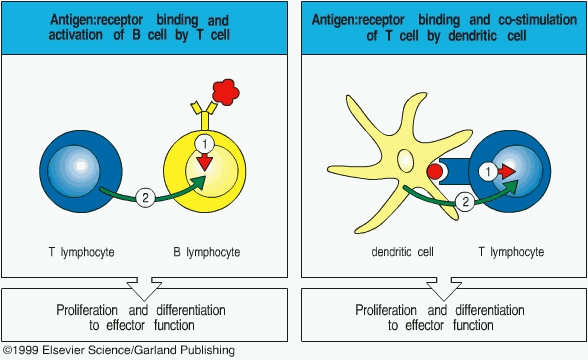
Occurring over the course of several days, the activation of naive T cells to an effector T cells capable of an immune response involves two steps.1 Firstly, there is a direct signal to the T cell upon recognition of an Ag.1 This direct signal is what has already been characterized and is called the Ag derived signal. Secondly, there is a co-stimulatory signal delivered by the DC, or any other APC, that primes the T cell.1 Most often, the co-stimulatory signal is a cytokine called B7.1 This pattern is typical of the interaction between T-cells and other APCs and the co-stimulatory signal is analogous to giving 00 status in the British Secret Service, better known as a license to kill. APCs also include macrophages and certain B cells and can generally be found in higher concentrations in the T cell zones of lymphoid tissues.1
DC in Negative Selection
Section 7-16 illustrates experimentally the role DC play in negative selection of T cells based on work done with chimeric (Gr. monster, genetically altered mutant organisms) mice. If skin grafts from mouse A are given to mouse B, then mouse B will reject the graft due to different MHCs.1 However, if DC from mouse A are given to mouse B in addition to the graft, then mouse B will accept the graft as the MHC presented from mouse A is no longer foreign.1 There are also other surface protein factors that could complicate this though, and this is illustrated in greater detail in the text.
Figures 7.6 and 7.13 do an excellent job of summing up the entire procedure. The entire process of selection, be it negative or positive, deals with changes of cell surface molecules. The presence or absence of surface molecules is wholly dependent on the random expression of T cell genes. The most primitive T cell is called CD3-4-8- (double minus). From random expression of genes coding for surface markers, either g:d+CD3+CD4-8- or CD3+pTa:b+4+8+ (double positive) thymocytes result.1 (Do not get hung up on the Greek characters and numbers; understand that they represent one or more protein markers; only the 4 and 8 should remind you of CD4 and CD8) The g:d+CD3+CD4-8- are discarded immediately because they are CD4 and CD8 negative and the g:d implies autoimmunity.1 Approximately 95% of the CD3+pTa:b+4+8+ (double positive) die via apoptosis for several reasons including the ability to recognize self antigen as determined by DC.1 The ~5% that remain lose either CD4 or CD8 to only express either CD4 or CD8 and become single positive.1 (eg. if a cell that is CD3+pTa:b+4+8+ (double positive) is proven to recognize foreign antigen then it might lose its CD8 character and become a CD4+ (single positive) lymphocyte)
DC Can Interact with B Cells and B Cell Products Directly
DC that migrate to the spleen will present Ag to B cells directly and therefore ultimately participate in humoral immunity as well as cell-mediated immunity.3,4,5 DC have also been indicated in participating in the isotype (variable region of the Fab) switching of Ab made by B cells.3,4,5
DC Control and Regulation (Homeostasis)
It is indicated that activated, mature T cells participate in a negative feedback loop by ceasing prolonged TH1 and TH2 activity by limiting and controlling the population of DC cells activating them.7 Ironically it seems that this negative feedback mechanism is dependent on the cytokines IL-4 and IFN-g used by DC in the first place to activate T cells.7
References
(1) Janeway, C.A., et al. Immunobiology: the Immune System in Health and Disease, 4th Ed. New York: Garland Publishing, 1999.
(2) Saudrais, C., et al. Intracellular pathway for the generation of functional MHC class II peptide complexes in immature human dendritic cells. 1998 Journal of Immunology 160(6): 2597-2607.
(3) Wykes, M., et al. Dendritic cells interact directly with na´ve B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response 1998 Journal of Immunology 161(3): 1313-1319.
(4) DuBois, B.; Massacrier, C., et al. Critical role of IL-12 in dendritic cell-induced differentiation of na´ve B lymphocytes. 1998 Journal of Immunology 161(5): 2223-2231.
(5) DuBois, B.; Barthelemy, C., et al. Toward a role of dendritic cells in the germinal center reaction: triggering of B cell proliferation and isotype switching. 1999 Journal of Immunology 162(6): 3428-3436.
(6) Tang, H.L.; Cyster, J.G. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. 1999 Science 284(5415): 819-822.
(7) Rissoan, M., et al. Reciprocal control of T helper cell and dendritic cell differentiation. 1999 Science. 283(5405): 1183-1186.
(8) Randolph, G.J. et al. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. 1998 Science. 282(5388): 480-483.
(9) Rovere, P., et al. Cutting Edge: Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. 1998 Journal of Immunology. 161(9): 4467-4471.